Clinical evidence
Macrostudy: Chlorine dioxide as an alternative treatment for COVID-19
Dr. Manuel Aparicio-Alonso, Carlos A. Domínguez-Sánchez* and Marina Banuet-Martínez Deparment of Natural Sciences, Jurica Medical Center, Queretaro, Mexico
Summary;
As of December 2019, the first case of COVID-19 was reported in Wuhan, China, and spread rapidly around the world. This disease has caused millions of deaths and to date there is no fully effective drug against this disease.
This study evaluated the negative and positive effects of chlorine dioxide (ClO2) as an alternative therapy for the treatment of COVID-19. Data were collected from the medical records of 1136 patients treated for COVID-19 with three different protocols of an aqueous ClO2 solution at a mean dose of 1.41 mg/kg.
The average time to symptom resolution was 4.84 days, and full treatment lasted 15.87 days. In addition, 6.78% of patients had mild and sporadic adverse reactions such as headache, dizziness, vomiting, diarrhoea and nausea.
There were no side effects that endangered the health of the patients.
Blood tests revealed no systemic abnormalities after ClO2 consumption. Liver enzymes, glucose, total cholesterol and triglycerides returned to normal at the end of treatment.
Without complications, 99.03% of patients were discharged.
Our findings show that, when used at the appropriate concentration and dosage, ClO2 as a solution effectively treats COVID-19 while being safe for human consumption.
Introduction
The disease reported in late 2019 (COVID-19), caused by the novel SARS-CoV-2 coronavirus, is characterised mainly by acute respiratory symptoms accompanied by fever, malaise, headache and, occasionally, digestive and nervous symptoms [1,2].
These symptoms are caused by excessive inflammatory responses [3,4] and coagulopathies due to endothelial damage caused by the SARS-CoV-2 Spike protein [5].
Since the early 2020`s, when the World Health Organization declared it, the COVID-19 pandemic has severely affected most countries in terms of morbidity and mortality, as well as in terms of the economic and social cost of the measures taken to curb the pandemic. One of the main challenges posed by this disease has been finding effective drugs to treat COVID-19 [6].
Chlorine dioxide (ClO2) is a soluble gas that is used in different countries to disinfect drinking water [7-9] due to its antimicrobial activity [10]. When both air and water are present, ClO2 is distributed between the two phases in an equilibrium relationship determined by temperature and atmospheric pressure [11].
ClO2 is known to denature tyrosine and tryptophan residues due to oxidation [10,12], and also has a modulating action on the immune system by inhibiting NF-kB transcription [13,14]. In this context, it is possible to assume that ClO2 can react with the SAR-CoV-2 Spike protein (composed of 54 tyrosine, 12 tryptophan and 40 cysteine residues) and inactivate the virus [15].
In addition, by neutralising reactive oxygen molecules and cytokines with ClO2 [16,17], it is possible to control the excessive inflammation associated with severe COVID-19 [1].
Although cysteine, tyrosine and tryptophan residues can also be found in human tissues, ClO2 is much less toxic to humans or animals than to bacteria and viruses due to its size selectivity [16,18] and due to the content of antioxidants such as glutathione in mammalian cells [19].
While ClO2 has been categorised as a hazardous compound when used for other applications in other forms and doses, due to some reported non-lethal side effects [19], it is important to consider that most of these cases are clinical reports of poisoning with other chemical substances like sodium chlorite (NaClO2) or sodium hypochlorite (Bleach, NaClO), and not ClO2.
Regardless, health authorities have issued misleading information that lacks scientific evidence on the toxicity of this chemical compound, thus affecting the development and implementation of ClO2 as a possible treatment for COVID-19.
To date, none of the drugs approved or cleared on an emergency basis by the Food and Drug Administration (FDA) to treat COVID-19 have demonstrated high effectiveness in reducing symptoms, hospitalisation and death. It is therefore critical to evaluate new compounds that can reduce the impact of the current pandemic, such as Ivermectin [20,21].
Evidence on the safety and efficacy of ClO2 is just beginning to be accepted in the medical community, although official regulatory institutions do not yet accept it. Here, we examined medical data from 1,136 patients with COVID-19 who used ClO2 solutions (CDS) as an alternative treatment. We evaluated the side effects produced by CDS consumption and its potential effectiveness in preventing serious illness and death.
Materials and Methods
Data collection: Baseline and clinical information Clinical records of 1,136 COVID-19 positive/suspected patients (treated by the same physician) who voluntarily requested home therapeutic management in Mexico were reviewed; these records spanned from 30 May 2020 to 15 January 2021.
Inclusion criteria for the clinical registries were as follows:
1) patients diagnosed by molecular testing (real-time reverse transcriptase (RT-PCR) for SARSCoV-2, antigen detection, specific immunoglobulin M (IgM) and immunoglobulin G (IgG) against SARS-CoV-2), computer-assisted computed tomography of the lungs, chest radiographs or a combination of clinical manifestations such as headache, fever, cough, sore throat, dyspnoea, malaise and fatigue [1,22];
2) patients informed about the benefits and possible side effects of ClO2 consumption before starting treatment and who had signed the informed consent form.
Variables collected from medical records were: sex, age, comorbidities, previous medications, date of onset, date of discharge or date of death, side effects following CDS consumption, millilitres of ClO2 consumed per day (“ClO2 per day”), partial oxygen saturation (SpO2), oxygen supplementation (O2 L/min) and COVID-19-like symptoms. In addition, six variables were calculated for each patient from the collected data: duration of COVID-19-like symptoms (“symptom days”), duration of treatment (“treatment duration”), millilitres of ClO2 consumed during treatment (“total ClO2“), ClO2 dose during treatment (“ClO2 dose”), ClO2 cost per day (“cost per day”) and total ClO2 cost during the entire treatment (“total cost”).
In addition, patients’ disease severity (mild, moderate or severe) was determined according to the parameters established in the Coronavirus Disease Treatment Guidelines (COVID-19) [23] and the interim algorithms for COVID-19 care of the Mexican Social Security Institute [24].
Two groups of patients were analysed:
1) Multi-drug patients: people taking drugs normally used to treat COVID-19 (Azithromycin, Dexamethasone, Ivermectin and Hydroxychloroquine) plus a chlorine dioxide solution, and
2) ClO2-only patients: people treated with a chlorine dioxide solution only. All patients were treated at home by their relatives or nurses according to the treating physician’s instructions.
Two types of oral aqueous ClO2 solutions at 3000 ppm (3 mg/ml) were used to treat COVID-19: Protocol C (ClO2 in 1000 ml of water, divided into ten 100 ml intakes given orally every hour, daily) and Protocol F (ClO2 in 500 ml of water, divided into ten 50 ml intakes given orally every 15 minutes, 1-5 times daily).
For intravenous use, Protocol Y (ClO2 in 500 ml of sterile 0.9% saline plus 5 ml of 10% calcium gluconate and 10 ml of 7.5% sodium bicarbonate, administered at an average rate of 70 ml per hour). All patients started treatment with Protocol F and, depending on the severity of the disease, were placed on Protocols C, F or Y until symptoms resolved. After the disappearance of symptoms, they continued on Protocol C as maintenance until treatment ended (14-21 days depending on disease severity).
The ClO2 in form of CDS used by patients for oral use was made by oxidising 28% sodium chlorite (NaClO2) and 4% hydrochloric acid (HCl) as an activator [19]. For intravenous use, ClO2 was produced by the membrane electrolysis method [9]. According to the instructions given to each patient, the ClO2 solution was kept in a closed bottle, protected from direct sunlight and kept below 11°C [19,25].
General physical condition of patients:
Symptoms reported voluntarily by patients were used to calculate the incidence of each symptom similar to COVID-19. Patients who died during the course of the disease were considered as unsuccessful treatment cases. The clinical condition of patients was assessed in a subset of 57 patients (mainly severe COVID-19 cases) for whom data on a complete blood count and a metabolic biomarker test were available before and after treatment.
As reference values, we used those reported for the healthy Mexican adult population [26,27].
Statistical analysis
An initial analysis of the data using descriptive statistics provided an overview of the baseline information of the patients included in this study. Prior to proper data analysis, the distribution of each variable was examined.
Variables deviated from a normal distribution and there was evidence of heteroscedasticity; therefore, we used Kruskal-Wallis tests to compare ClO2 values per day, symptom days, treatment duration, total ClO2 administered, ClO2 dose, cost per day and total cost between disease severity (mild, moderate and severe).
Duration of symptoms and duration of treatment among comorbidities were also analysed using Kruskal-Wallis tests. The Wilcoxon signed-rank test was used to compare symptom days and treatment duration between multidrug and ClO2-only patients, as well as to compare results between blood tests (complete blood counts and metabolic biomarker panel tests) before and after treatment.
Treatment efficacy was assessed by dividing unsuccessful cases by the total number of patients. A log-transformed linear regression model was fitted to analyse the association of treatment duration to end of symptoms with SpO2 and O2 L/min. Logistic regression was fitted to analyse the association of age, sex and comorbidities with disease severity.
A p-value of p <0.05 was considered statistically significant. Continuous outcomes were measured as the mean difference and 95% confidence intervals (CI). To reduce reporting bias in this study, the treating physician was not involved in the digitisation or statistical analysis.
All analyses were performed using Rv.3.6.1 [28].
Ethical approval The Ethics Committee of the Jurica Medical Centre waived the need for ethical approval and the need to obtain consent for the collection, analysis and publication of retrospectively collected data because this was a non-interventional study in which information was captured from old medical records, maintaining the anonymity of each individual and because all patients signed an informed consent prior to treatment. Data availability Data sets used and analysed during the present study are available upon reasonable request from the corresponding author.
Results Descriptive analysis of patients Primary data were collected from 1,136 patients (Table 1) in 30 states of Mexico, mainly in Queretaro (53.07%), Mexico City (10.22%) and Jalisco (5.11%). Of the entire sample set, 487 (42.87%) patients were diagnosed as COVID-19 positive by molecular testing or diagnostic imaging; the remaining 649 patients (57.13%) were diagnosed due to COVID-19-like symptoms. At the end of treatment, 213 (18.75%) patients underwent specific antibody testing for SARS-CoV-2, and 154 (72.30%) tested positive (93 for IgG and 61 for IgM).
Patients were classified according to severity of illness into three groups: mild, moderate and severe, according to symptoms and SpO2. The study included 551 (48.50%) males, 525 (46.21%) females and 60 (5.28%) for whom there was no information on sex.
Severity was associated with sex (x2=16.89, df=2, P=0.0002); men were 1.8 times more likely than women to develop a severe case of COVID-19 (RR=1.8, 95% CI: 1.33-2.42, P<0.001). The mean age was 46.72 (range 1-93) years, and COVID-19 was more prevalent in the 40-49 and 50-60 age groups (19.01%, 21.04%; respectively).
The risk of developing severe disease was determined by age (x2=82, dF=7, P<0.0001), increasing by 4% for each year of life (OR=1.04, 95% CI: 1.03-1.05, P<0.001).
The risk of developing more severe disease was highest after the age of 30 years (Figures S1 and S2). A total of 25 different symptoms were reported by patients, with the most frequent symptoms (table S1) being headache (49.65%), malaise (44.45%), sore throat (37.41%), fever (22.89%), dry cough (17.34%), weakness (14.70%), chest pain (12.32%), dyspnoea (9.5%), anosmia (9.15%) and ageusia (8.71%). The average duration of symptoms was 4.84 days (95% CI: 4.32-5.36 days) and differed according to severity of illness (mild: 2.52 to 3.33 days, moderate: 7.89 to 12.21 days and severe: 6.73 to 9.95 days; Kruskal-Wallis, x2=234.89, df=2, P<0.001) (Table 2).
Chlorine dioxide treatment A total of 1,067 (93.96%) patients were discharged after 15.87 days (95% CI: 15.35-16.39 days) of treatment, 59 (5.19%) dropped out after 11.43 days (95% CI: 7.98-14.88 days), and 10 (0.93%) were hospitalised after 8.6 days (95% CI: 2.08-15.11 days) of treatment, where they died.
The calculated effectiveness of ClO2 treatment was 99.07% (1,057 of 1,067 patients survive).
Of the total patients, 77 (6.78%) reported mild and sporadic side effects following ClO2 ingestion: headache (2.20%), diarrhoea (1.58%), gastritis (1.32%), dizziness (1.14%), nausea (1.05%), vomiting (0.44%), rash (0.44%), sore throat (0.26%), myalgia (0.18%), colitis (0.18%), tachycardia (0.09%) and chills (0.09%).
A total of 666 patients (58.63%) were treated exclusively with CDS, and 470 patients (41.37%) were treated against COVID-19 with five or more drugs in addition to CDS (Table S2). The duration of symptoms in those patients treated with CDS alone was shorter compared to those treated with multiple drugs (95% CI: 2.77-3.75 days vs. 7.33-8.97 days, respectively; Wilcoxon rank sum test, P<0.001).
Depending on the severity of the disease and the evolution of the patients, different protocols were used during treatment. Nine hundred and sixty-one (84.59%) patients used Protocol C, 474 (41.72%) used Protocol F and 42 (3.70%) used Protocol Y. Protocol C was widely used in mild and moderate patients, Protocol F in severe patients and Protocol Y was mainly used as adjunctive treatment in severe cases (Figure 1). The average daily dose used to treat patients with COVID-19 was 1.41 mg/kg (95% CI: 0.97-1.85 mg/kg), corresponding to 32.95 ml per day (95% CI: 22.72-43.18 ml/day) for 15.87 days (95% CI: 15.35-16.39 days). However, for each protocol (C, F and Y), the dose and days of consumption varied according to the severity of the disease (Table 3).
Overall, patients were treated with the following doses and duration: Protocol C (mean: 20.16 ml per day [95% CI: 18.94-21.37 ml/day] for 8.99 days [95% CI: 8.46-9.52 days]), Protocol F (mean: 39.13 ml per day [95% CI: 35.34-42.92 ml/day], 2.75 times per day [95% CI: 2.53-2.97 intakes/day] for 5.36 days [95% CI: 4.74-5.98 days]); and Protocol Y (average: 89.92 ml per day [95% CI: 46.65-133.19 ml/day] for 1.77 days [95% CI: 1.39-2.14 days] in 2.12 infusions per day [95% CI: 1.64-2.60 infusions/day]).
Nine patients gargled with a 0.015% aqueous solution made of 5 ml ClO2 in 100 ml water in case of sore throat or nasal congestion.
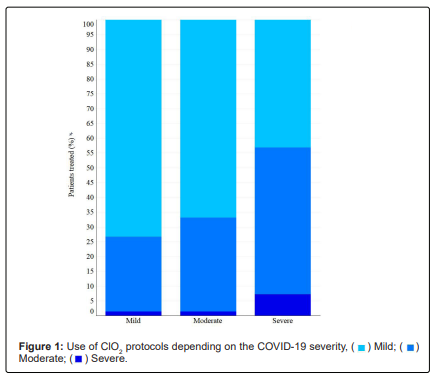
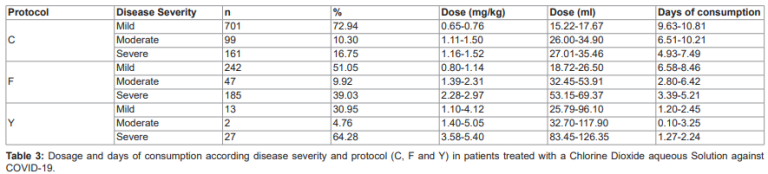
There were differences in treatment duration (Kruskal-Wallis, x2=30.42, df=2, P<0.001), ClO2 dose (Kruskal-Wallis, x2=116.62, df=2, P<0.001) and ClO2 per day (Kruskal-Wallis, x2=72.20, df=2, P<0.001) between patients with mild, moderate or severe COVID-19 (Table 3).
The mean ClO2 consumed by patients during the whole treatment was 557.94 ml (95% CI: 390.19-725.66), and was different in each severity (mild: 309.83-337.38 ml, moderate: 518.77-619.19 ml and severe: 733.67-828.79 ml; Kruskal-Wallis, x2=52.05, df=2, P<0.001). The estimated mean duration of symptoms is 2.82 (95% CI: 1.16, 4.47, P<0.001) days less per mg/kg ClO2, adjusting for severity.
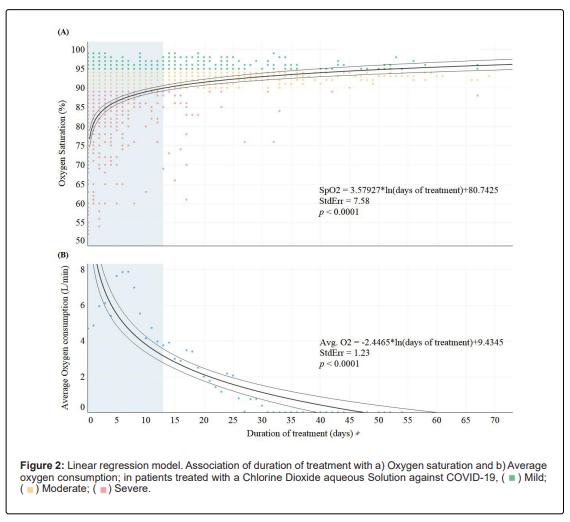
Patients started treatment with an average SpO2 of 86.05% (95% CI: 85.12-87.17%), with blood oxygen increasing each day of treatment. In total, 126 patients (101/251 [40.24%] with severe symptoms, 21/109 [19.27%] with moderate symptoms and 4/776 [0.51%] with mild symptoms) used supplemental oxygen (mean: 5.77 litres per minute [95% CI: 5.18-6.36 L/min] for 4.32 days [95% CI: 3.37-5.27 days]). Between days 7-8 after initiation of treatment, 90% of patients reported an increase in SpO2 above 90% and one week later above 95%, at a rate of SpO2=3.58ln(treatment duration) (Figure 2a).
Days to reach 90% SpO2 did not differ between patients who received supplemental oxygen and those who did not (95% CI: 7.53-9.47 days, P=1.00); however, it took almost five days less for patients on supplemental oxygen to have an SpO2 of 95% compared with those who did not (95% CI: 12.53-14.47 days vs. 18.52-20.48 days, P=0.004). Oxygen supplementation decreased at a rate of -2.45ln(treatment duration) (Figure 2b). Furthermore, duration and amount of oxygen administered differed by severity (Kruskal-Wallis, x2=9.6382, df=2, P=0.008; x2=16.89, df=2, P=0.002; respectively).
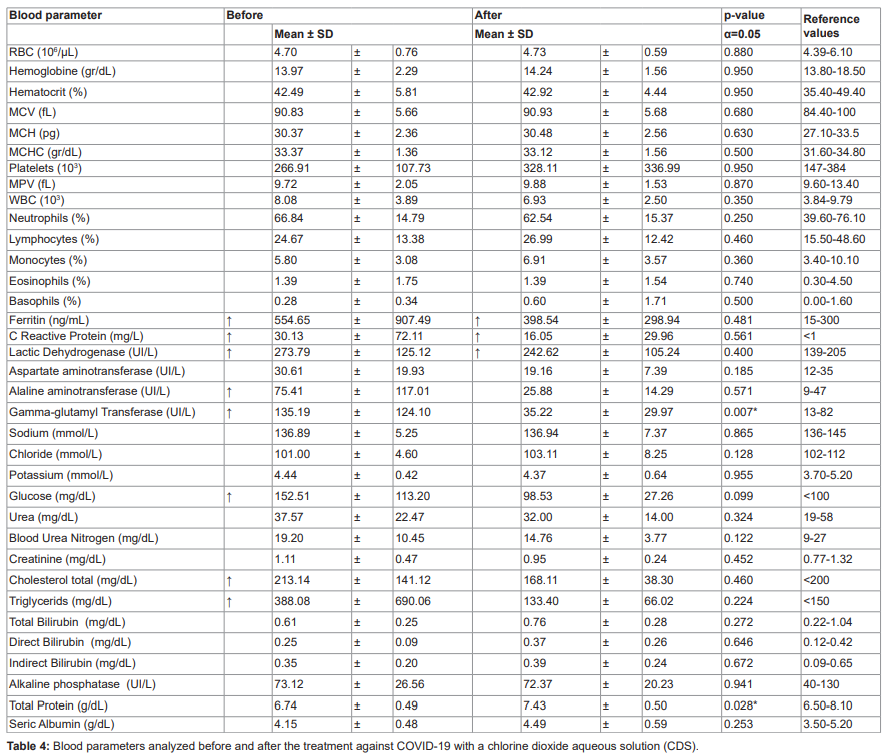
All haemogram parameters were within normal ranges before and after ClO2 treatment. All patients, for whom blood metabolites were available, started treatment with elevated levels of ferritin, C-reactive protein, lactate dehydrogenase, alanine aminotransferase, gamma-glutamyl transferase, glucose, total cholesterol and triglycerides. After ClO2 consumption, most of these parameters decreased to normal physiological values. The exceptions were serum ferritin concentration, C-reactive protein and lactate dehydrogenase, which decreased but did not reach normal levels during the duration of the study (table 4).
Days to reach 90% SpO2 did not differ between patients who received supplemental oxygen and those who did not (95% CI: 7.53-9.47 days, P=1.00); however, it took supplemental oxygen patients almost five days less to reach 95% SpO2 compared to those who did not (95% CI: 12.53-14.47 days vs. 18.52-20.48 days, P=0.004). Oxygen supplementation decreased at a rate of -2.45*ln (treatment duration) (Figure 2b).
Furthermore, the duration and amount of oxygen administered differed according to severity (Kruskal-Wallis, x2=9.6382, df=2, P=0.008; x2=16.89, df=2, P=0.002; respectively). All parameters of the complete blood count were within normal ranges before and after ClO2 treatment. All patients, for whom blood metabolites were available, started treatment with elevated levels of ferritin, C-reactive protein, lactate dehydrogenase, alanine aminotransferase, gamma-glutamyl transferase, glucose, total cholesterol and triglycerides.
After ClO2 consumption, most of these parameters decreased to normal physiological values. The exceptions were serum ferritin concentration, C-reactive protein and lactate dehydrogenase, which decreased but did not reach normal levels during the duration of the study (table 4).
Discussion This retrospective study collected data from 1,136 people who used a CDS as a treatment for COVID-19.
We found ClO2 to be a safe and effective treatment for COVID-19 patients, which, regardless of severity, reduced symptoms in 99.03% of cases.
Comorbidities, age and gender were associated with the severity of COVID-19 presented by the patients (Appendix 1). Because the effect of ClO2 depends not only on its concentration but also on contact time [19], patients were treated with a CDS using different protocols (varying in dose and intake intervals) according to disease severity, with an average dose of 1.41 mg/kg per day (range 0.67 to 5.40 mg/kg/day). In addition, nine patients reported gargling with 0.015% CDS.
It has been proposed that the mouth and oropharynx can be disinfected by regular rinses with a microbial solution such as povidone-iodine [29] or a CDS [17,19,30] to significantly reduce the viral load in the mouth and upper respiratory tract.
The treatment doses used in this retrospective study were within the safety limits reported for human use [7,31,32]. In addition, the doses used were below the “Lowest Observed Adverse Effect Level” (LOAEL); being eight times lower than the doses at which adverse effects occur and at least 30 times lower than the lethal dose 50 (LD50=94 mg/kg; World Health Organization, 2002).
Oral intake of ClO2 at concentrations of 5 mg/L for 12 weeks has not been shown to have harmful effects in humans [7,31]. Recently, it has been shown that at a dose of 0.6 mg/kg, ClO2 has prophylactic potential against COVID-19 without causing moderate or severe adverse effects in most patients; in those who reported side effects (1.12%), symptoms were mild and sporadic [33,34]. In addition, ClO2 has been shown to be a size-selective antimicrobial, and at appropriate concentrations, it can be used in animals and humans due to its inability to penetrate tissues [16,18,19].
Compared to other drugs, COVID-19 patients taking ClO2 had a shorter recovery time than reported for other treatments. It is important to note that the duration of symptoms in patients treated exclusively with ClO2 was less than half compared to patients treated with multiple drugs (3.26 days versus 8.15 days, respectively).
Concurrent use of multiple drugs has been associated with increased mortality among male COVID-19 patients and increased rates of acute kidney injury and adverse drug reactions [35].
It is recommended that clinical trials be designed with detailed follow-up to assess the effect of Chlorine Dioxide on recovery time and its interactions with other drugs.
In this study, 6.78% reported mild transient side effects following CDS ingestion, including headache, diarrhoea, gastritis and dizziness; side effects similar to those reported in a previous study [34]. In these 77 patients, the dose was halved immediately after the onset of symptoms. Subsequently, a gradual increase was made until the treatment dose was reached.
After this adjustment, no patients reported adverse reactions again. Patients treated with intravenous CDS reported no side effects. Our results show that ClO2 at the dose used is safe and has no serious side effects, even if used at higher doses. Blood tests also corroborate the absence of adverse effects, as most of the measured parameters were found to be within normal ranges after treatment.
Ferritin, C-reactive protein and lactate dehydrogenase were above standard limits. However, these analytes were lower compared to baseline. In addition, liver enzymes, glucose, total cholesterol and triglycerides were lower at the end of treatment.
The percentage of patients treated with CDS who were discharged (99.03%) was higher than that reported in other studies (ranging from 85% to 92.3%; Beigel 2020; Heras 2021; Rajter 2021). In that sense, our retrospective study warrants prospective cohort studies or blinded randomised controlled trials to adequately compare the effect of ClO2 with that of other drugs.
Due to the limited published evidence on ClO2 as an alternative treatment in humans, conducting such studies in a controlled setting is urgent, relevant and necessary, especially given that in our study the average duration of symptoms in patients treated with ClO2 was 4.84 days; almost 9 days less than the national average [36-38], and more than 20 days less than the average recovery time of patients in India, a country with similar socioeconomic conditions and demographic structure to Mexico [39]. In addition, 92.01% of patients in our study were cured before day 10 of treatment, whereas in the aforementioned study only 4% were cured in the same time.
Recovery time was also shorter than that of patients in Belgium, Hong Kong and the UK, where they remained hospitalised for 5.9 days, 4.41 days and 5.14 days, respectively [40,41]; in Canada and Brazil, recovery time is close to 14 days, while in Japan the average period is less than 14 days [38]. A large meta-analysis (including 25 countries) revealed that recovery time in patients with COVID-19 ranged from 5 to 29 days [6], implying that the use of ClO2 could considerably reduce the duration of symptoms in patients with COIVD-19, even in those with severe symptoms.
Patients started treatment with an average SpO2 of 86.05%, a condition known as severe hypoxia [23]; 129 patients received an average of 5.77 litres per minute of oxygen for 4.32 days. After one week of ClO2 consumption, 90% of patients had moderate hypoxia (SpO2 between 90% and 94%), and two weeks later, patients were no longer hypoxic. Interestingly, the 90% SpO2 threshold was reached in both oxygen-supplemented and non-oxygen-supplemented patients within the first seven days of treatment. However, severe patients without oxygen supplementation took almost five days longer to reach an SpO2 above 95% than those without oxygen supplementation.
From the first intake of CDS, there was an increase in the patients’ blood oxygen levels, which improves the physiological response and reduces the patient’s anxiety about hypoxia [42]; however, oxygen supplementation was essential, mainly in patients with severe disease, as it helped to speed up the recovery of sick people. Further studies are needed to understand the mechanism by which ClO2 improves blood oxygen concentration.
A major benefit of ClO2 treatment is that patients can be treated at home without the need for hospitalisation. This prevents the occurrence of common bacterial or fungal infections in the ICU (average 40.7%), which have been significantly associated with death (OR 2.7, 95% CI 1.2-5.9, P=0.015) [43-58].
Home treatment increases the likelihood of patient survival and additionally avoids the collapse of health systems, especially in low- and middle-income countries [6], such as Mexico. Treatment of COVID-19 generates significant costs in public hospitals and is very expensive in private hospitals, so a large proportion of the population cannot access private care.
Limitations: While exciting, we are aware that our study has some limitations. The first is that it is a retrospective observational study, which means that conclusive evidence on the effectiveness of ClO2 cannot be established because we were only able to use information from patients’ medical records and had no control over study variables.
Secondly, there is a possible misinformation bias because relatives or patients provide the baseline and clinical information. Thirdly, it was impossible to establish with certainty that all patients had COVID-19 because diagnostic tests had not been performed for all patients.
However, 72.30% of patients who underwent antibody testing had developed IgG or IgM against SAR-CoV-2. Fourth, due to the lack of additional information, the interpretation of our findings might be at least partially confounded (e.g. differences in dietary habits, correct treatment follow-up and ClO2 quality). These and other variables should be taken into account in future studies.
Conclusion;
This is the first study to examine the adverse effects and benefits of a chlorine dioxide solution as an alternative treatment for COVID-19. Side effects from consuming chlorine dioxide are rare, 6.78% of patients reported side effects and these were mild, transient and not life-threatening.
Blood tests revealed no systemic changes after chlorine dioxide consumption; moreover, several initially elevated blood parameters decreased and became normal after chlorine dioxide treatment. From the first intake, chlorine dioxide improves the oxygen concentration in the blood, which improves the physiological response.
Patients treated with chlorine dioxide alone had fewer days with symptoms compared to those treated with multiple medications. 99.07% of the treated patients were discharged without any health problems. Our findings indicate that when used correctly, chlorine dioxide as a solution is safe for human consumption at the appropriate concentration and dosage. This study demonstrates a high level of safety and efficacy of chlorine dioxide in the treatment of COVID-19.
These findings warrant randomised controlled trials to assess its efficacy against SARS-CoV-2. Such a trial could pave the way for further research into the potential use of new compounds to solve current and future public health problems, which is, after all, the goal of the World Health Organisation and other health authorities.